Aldehydes In Sugars Are So Silently Destructive To Our Bodies.
But most of us don't even know what an aldehyde is, in the first place.
It’s quite interesting to note these days what we can eat and what we shouldn’t be eating. Even if the food product is actually edible, the metabolites may not be regarded as safe.
We know a few things about what not to consume in overly large quantities. Sugar, we know, isn’t that great. Alcohol, we know that we shouldn’t be drinking too much of. Polyunsaturated fats (or even partially hydrogenated fats) aren’t that great either.
But wouldn’t it be interesting to know that why they’re not good for health can be distilled down to the exact same chemical reaction?
Now, sugary foods are rich in sugars such as glucose and fructose.
Glucose, on its own, can exist as an aldehyde, or it can be converted into glyceraldehyde in the body by first converting it into fructose (it therefore goes without saying that fructose can also be converted into glyceraldehyde).
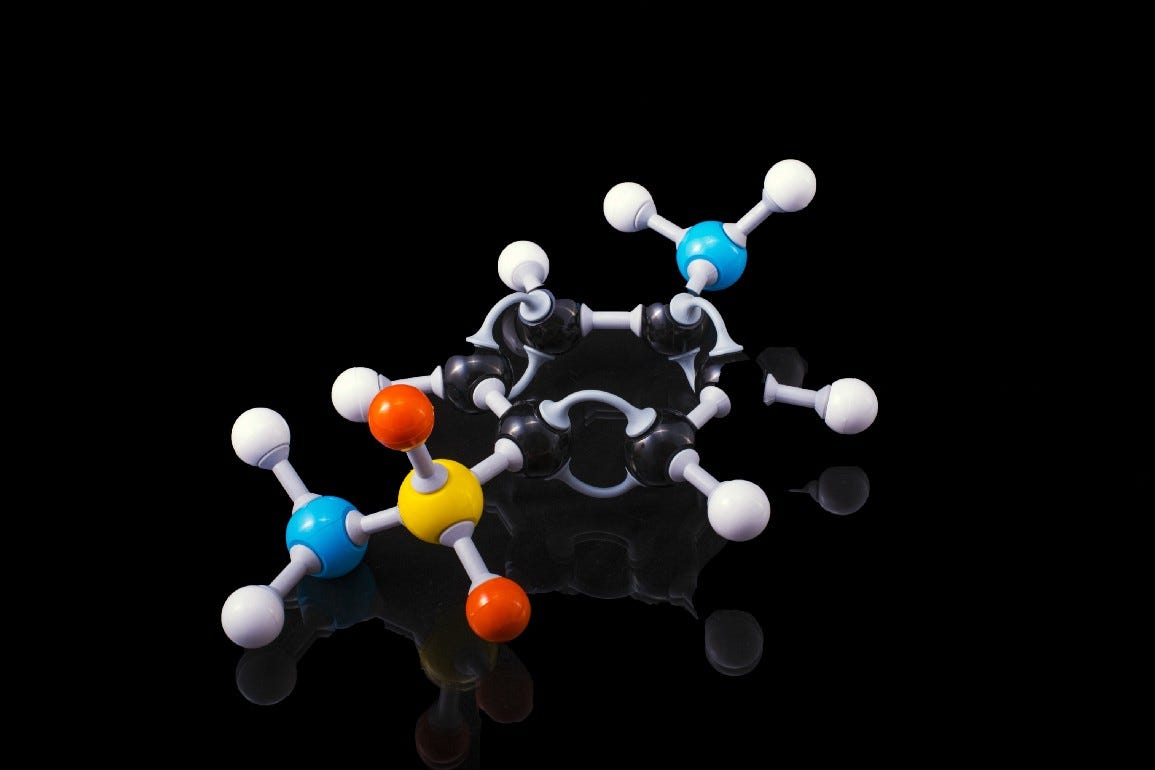
Aldehydes can react with amines, and that’s what we see in the Maillard reaction when we see the browning of potato chips, for instance.
Of course, the proteins in our body and our cellular DNA all do contain amines, and all these aldehydes derived from excessive sugar consumption can react with them.
That’s the thing that isn’t great for our health, because we’d see the proteins in our body and the cellular DNA components becoming damaged — in some cases, the damage can be irreversible, too.
We call this reaction a “glycation” reaction, though it’s just about an amine reacting with an aldehyde — such is the nature of haemoglobin proteins reacting with glucose to form HbA1c. And of course, the glycated proteins tend to lose their biological functions:
Why Diabetics Are Likely To Face Future Issues With Limb Amputations.
Apparently, maggot debridement therapy is a mode of treatment for diabetic patients with foot injuries. According to this article:
So there we have the aldehyde-amine reaction in full force.
But of course, we can see that happening in alcohol consumption too.
Keep reading with a 7-day free trial
Subscribe to The Biochemistry Of Human Health to keep reading this post and get 7 days of free access to the full post archives.